|
|
 |
|
|
 |
|
How does salt dissolve in water?
|
Common salt, the sort we put on food, is a chemical compound called sodium chloride. Salt crystals are made up of molecules of sodium chloride. Each molecule has one atom of sodium chemically bonded to one atom of chlorine. The atoms take the form of ions, which have an electric charge. Sodium ions have a positive charge and chloride atoms have a negative charge. When salt dissolves in water, the ions separate and become surrounded by molecules of water.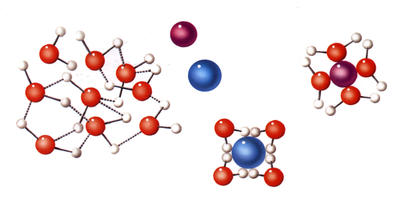 | | Salt dissolves quickly in warm water, and as it does so its ions separate. |
|
| |
Previous:
Back
|
Book:
1001
|
Section:
Science and Technology
|
Chapter:
Chemistry
|
|
|
|
 |
|
|