|
|
 |
|
|
 |
|
How are items electroplated?
|
When certain chemicals are dissolved in water they split into ions. Copper sulphate, for example, splits into copper ions and sulphate ions. If two electrodes are dipped into the solution and connected to a battery, the ions move toward the electrodes. Copper ions move to the electrode connected to the negative terminal (the cathode). As a result, the cathode is covered, or electroplated, with a layer of copper metal.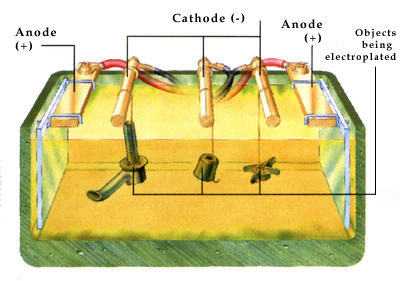 | | Parts of a tap are chromium electroplated by being wired to the cathodes of a plating tank. |
|
| |
Previous:
Back
|
Book:
1001
|
Section:
Science and Technology
|
Chapter:
Electricity and Magnetism
|
|
|
|
 |
|
|